Memphis Medical Device Start-up Utilizes Region’s Strengths to Launch New Surgical Solution for “Hammertoe” Deformity
When podiatric surgeon Scott Roman, DPM, of the Ankle and Foot Centers of Georgia in Atlanta designed a new device for surgical repair of proximal interphalangeal joint flexion deformity, the often painful condition known as “hammertoe,” a professional associate urged him to contact Patrick Mullaney for assistance in launching the device. After all, Roman needed to stay focused on his thriving practice, and product development is a complex and time-consuming process. Mullaney has over 20 years of experience selling orthopaedic implants and successfully commercializing other medical device technologies. Additionally, Mullaney is based in Memphis, Tennessee, a region which has been a hotbed of medical innovation for decades.
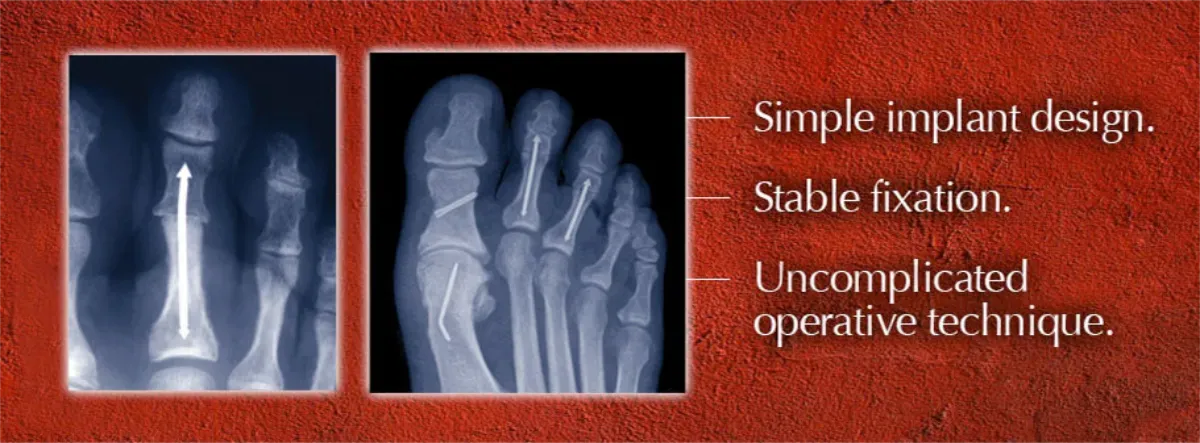
Mullaney and Tom Twardzik, a professional associate with 25 years in the industry, co-founded Arrowhead Medical Device Technologies, LLC, to launch Roman’s device, the ARROW-LOK™ Digital Fusion System — a toe implant that was designed to be effective, easy-to-use and more comfortable for patients than traditional wire devices. The outstanding market potential for hammertoe fixation made the ARROW-LOK concept easy to sell to investors and potential users.
Doctors began using Roman’s device in patients in the fall of 2010 following 510(k) FDA clearance. Surgeon feedback led to the development of a “second generation” implant design that was cleared by the FDA in October 2011. The ARROW-LOK Digital Fusion System has since been selectively launched in over 20 markets across the country.
Launching a new medical device is “a daunting task,” Mullaney says. “You have to finalize the design, implement production, develop a compelling message, and create a distribution network. Your people on the street personally present the product to the surgeons and support its use in the OR. Sales reps are the actual human contact you have with the users, with hospitals and surgery centers. We are very selective about who represents ARROW-LOK technology, and work through a network of independent agents in strong markets. That’s allowing us to rapidly gain acceptance of the ARROW-LOK system among leading podiatric and orthopaedic surgeons.”
Commenting on the value of the company’s location, Twardzik says, “Memphis is a very attractive area for medical device companies. It’s centrally located which makes it ideal for distribution, and the medical device industry is already well established here. In addition to several major medical research centers, we have some of the world’s leading device manufacturers in this area, the support of the Memphis Chamber of Commerce and the Memphis Bioworks Foundation, and an exceptional pool of experienced professionals with expertise in regulatory affairs, quality assurance, intellectual property, finance, product development, engineering, logistics and transportation. We literally have a comprehensive collection of people with specialized skills all right here.”
Arrowhead plans to continue developing new applications for the ARROW-LOK technology to make the product even more attractive to specialists routinely performing foot surgery across the United States. In its report “US Market for Small Bone & Joint Orthopedic Devices,” published June 2011, iData Research estimated that 500,000 hammertoe surgeries are performed annually in the United States.
“I’ve had several patients that have been putting off surgery for years because they didn’t want to go through the experience with the external wires, the most common manner of fixation,” Roman says. “The ARROW-LOK system is a comparatively anxiety-free postoperative experience for them. As we bring the device to doctors across the country, the patient population will become more aware of the product, and discover that they can have hammertoe surgery without wires protruding from the ends of their toes. That’s when they will begin requesting to be treated with a product like the ARROW-LOK device.”

Founded in August 2010, by Patrick Mullaney, President, and Tom Twardzik, Vice President, Sales and Marketing, Arrowhead Medical Device Technologies, LLC, specializes in rapidly developing, manufacturing and distributing medical devices utilized in the treatment of musculoskeletal conditions. The headquarters is located just outside of Memphis, Tennessee, a region recognized as one of the world’s premier sites for musculoskeletal medical device innovation. Arrowhead received clearance to market its initial product, the ARROW-LOK™ Digital Fusion System in October 2010, performed the first surgeries in December 2010 and commenced unrestricted distribution in February 2011. Arrowhead plans to expand its product lines to include additional sizes and applications for the ARROW-LOK technology. In addition, the management of Arrowhead is actively seeking innovative medical device technologies that serve to improve patient care in an effective and affordable manner. (www.arrowheaddevices.com)
Established in 2001 as a nonprofit 501(c)(3), the Memphis Bioworks® Foundation is bringing together public, private, academic and government entities in a collaborative effort to change the Memphis bioscience landscape. The Foundation is leading initiatives to expand upon the community’s current bioscience niches and demonstrated areas of leadership, and to gain international recognition of Memphis as a center for the development and commercialization of bioscience technology. (www.memphisbioworks.org)
The Greater Memphis Chamber is a diverse organization of civic, community, business and professional leaders whose collective mission is to achieve economic development by strengthening existing business and serving as a catalyst for new commerce and economic development, community development by fostering partnerships that build a qualified workforce linked to targeted industry, efficient infrastructure and an environment that stimulates growth, and member development by accelerating our members’ growth and success by connecting people, programs and resources. (www.memphischamber.com)
The Ankle and Foot Centers of Georgia is a long-established group of Board Certified podiatric physicians who are highly skilled innovators in the field of foot and ankle medicine. Founded in 1982 on the principles of technical excellence and personal concern for their patients’ health and well-being, the Centers’ doctors strive to provide patients with the latest available treatment options from conservative care to surgical intervention. (www.ankleandfootcenters.com)
Attention Patients
Would you like your surgeon to use the ARROW-LOK Digital Fusion System for your hammertoe correction?
Call us at 470-419-1429 or email us and we will direct you to an ARROW-LOK surgeon.